1. Device Closures of Holes is Effective, Safe and Time Tested Procedure. 2. It is painless and scar less.
Patent Ductus Arteriosus (PDA) Occluder
Technical principles
The occluder is made of self-expandable nitinol wire mesh, which is able to comply with the shape of defect parts, so it can be fixed well and do not affect tissues around. Meanwhile, with the flow blocking material sewing in the wire mesh, thrombus can be formed quickly that the endothelial cells could cover on the surface of the occluder to close the shunt completely.
Basic product structure
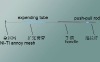
1. Self-expandable single-disc occluder, mushroom-shape.
2. Consisted of metal wire mesh made of nickel-titanium alloy, flow blocking material, stitches, front termination point and back termination point.
3. No adhesion between each layer of flow blocking material.
Product specification
Complete ranges of specifications from P0406 to P2426, for detail informations, please contact with us by email, fax,or phone.
Characteristics:
1) The metal mesh of the closure is knitted by imported nitinol wire(different size with different thickness), which has good memorability and biocompatibility.
2) Flow blocking material is good at stopping abnormal bleeding, and it is very thin that decrease the diameter of delivery catheter, which could reduce vascular injury from the operation.
3) Complete product sizes make the occluder more convenient for clinical use.
Applied scope
1)Age of the patients should be older than or equal to 6 months, whose weight is heavier than or equal to 4kg.
2)Patients are diagnosed as PDA by clinical diagnosis, electrocardiogram, chest film or transthoracic echocardiography, and are considered suitable to deploy closure treatment, with no contraindications.
3)Meet either criterions listed below:
A. The shunt should be from left to right, not associate with other cardiamorphia must be surgical operated. The narrowest PAD diameter should be bigger than 2.0mm but smaller than 14.0mm.
B Patients with residual shunt after surgery.
4)Whole body in good condition.
5)The patients are willing to do the surgery and sign the information consent form.
Patent Ductus Arteriosus (PDA) Closure/Occluder