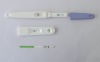
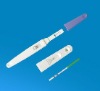
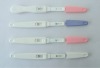
FSH rapid diagnostic test Kit strip CE Approved specimen: urine only sensitivity: 25miu/ml certificate: CE&ISO
FSH rapid diagnostic test Kit CE Approved
Human Follicular Stimulating Hormone Rapid test kit
fsh medical diagnostic test strip kits
specimen: urine onlysensitivity: 25miu/mlcertificate: CE&ISO
Introduction
One step FSH urine test is a test card for the determination of FSH(human folliculay stimutating hormone) concentration in urine specimens. test results are read visually without any instrument. The sensitive reaches 25mIU/ml.
Feature
format: strip, cassette and midstreamspecimen: urine onlysensitivity: 25miu/mlcertificate: CE&ISO
Test procedure
Test strip and specimens should be brought to room temperature(20-30°C) prior to testing.1. Remove the test device from its foil pouch and use it as soon as possible.2. Immerse the test strip into the urine sample with the arrow end toward the urine for about 10 seconds. Do not immerse past the marker line. Take the strip out and lay the strip flat on a clean, dry, non-absorbent surface.3. Wait 10 minutes and read results. It is important that the background is clear before the result is read. Results obtained after 30 minutes should be considered invalid.
Interpretation of results1. clear band on the test region. It means FSH in the sample is negative.2. positive: two distinct purple bands appear on the control and test region. It means FSH in the sample is positive.3. negative: one line on the control line region, it means the FSH in the sample is negative.4. invalid: no visible red at all, it means the test is fail.
FSH strip rapid diagnostic test Kit FSH strip CE Approved